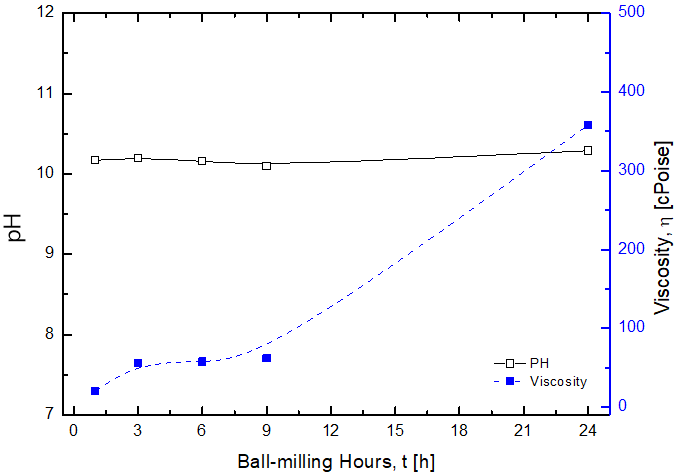
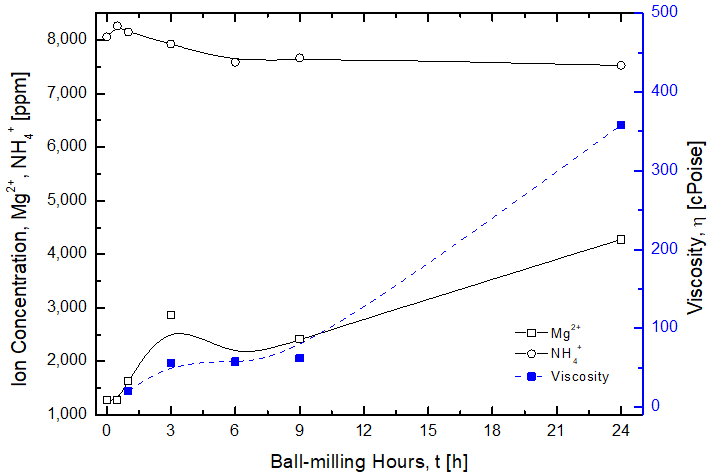
Viscosity Increase of Mg(OH)2 Slurry with Ball-milling
(1) Viscosity Increase of Mg(OH)2 Slurry with Ball-milling, eventually make spray drying be unable
(1w% Poly carboxylic Ammonium Salt)
(2) This viscosity increase is believed to be due to concetration increase of Mg2+ with ball-milling
(Below is the result of ion chromatography analysis conducted by Center for Research Facility, YU)
(3) 31vol. % aqueous Mg(OH)2 slurry, that can be sprayable, can be prepared with the dispersants
(1w% Poly carboxylic Ammonium Salt + 1 w% NH4OH)